
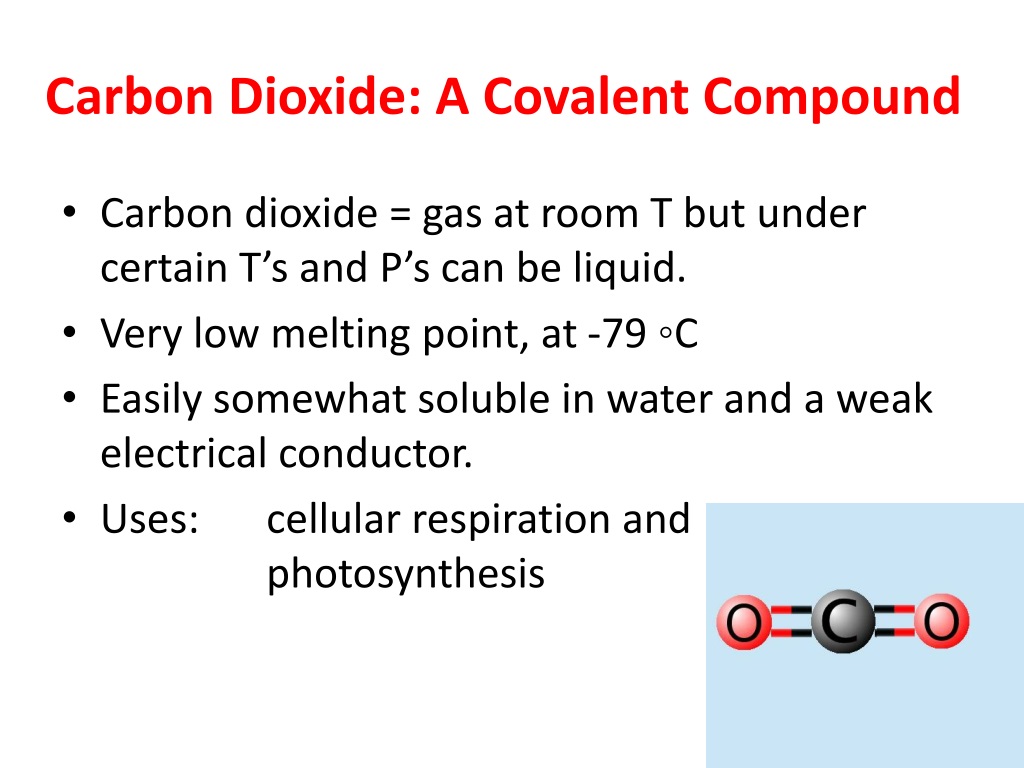
So the overall total number of electrons should be 2, this is the electron region number.Įrror message | View complete answer on How many single covalent bonds are in CO2?Įrror message | View complete answer on Is CO2 a single double or triple covalent bond?ĬO2 is a linear molecule O=C=O. The CO2 molecule has 2 double bonds so minus 2 electrons from the final total. Since they do not contain ionic bonds, they cannot be the correct answer to the problem.Įrror message | View complete answer on Is CO2 single or double bond? Therefore, they will contain only covalent bonds. Another method is by calculating the difference in electronegativity, we can determine the polarity of the molecule. Carbon and oxygen are non-metals, thus we know carbon dioxide is a covalent compound. Is CO2 ionic or covalent : The covalent compound is formed by two non-metal atoms, in case of CO2 compound formation, carbon and oxygen are non-metal while the ionic compound is formed by a metal atom and a non-metal atom.Įrror message | View complete answer on Does CO2 contain both ionic and covalent bonds?įirst, three of the answer choices - CO2, H2CO3, and H2O - contain only nonmetals. CO2 is made up of: - 1 carbon atom, - 2 oxygen atom. Carbon and oxygen are non-metals, thus we know carbon dioxide is a covalent compound.5 ngày trướcĮrror message | View complete answer on What type of compound is CO2?Ĭarbon dioxide is a one-carbon compound with formula CO2 in which the carbon is attached to each oxygen atom by a double bond.Įrror message | View complete answer on Why is CO2 not covalent?
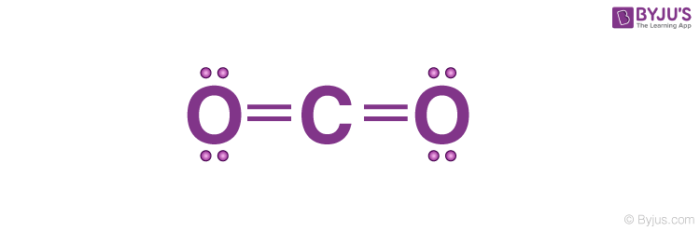
There are four covalent bonds in one molecule of carbon dioxide. The difference in the electronegativity between these two atoms is equal to 1, thus classifying the bonds in sulfur dioxide molecular as covalent.Įrror message | View complete answer on Is CO2 a covalent compound?Ĭarbon dioxide is made up of one carbon atom, two oxygen atoms. The electronegativity of sulfur is equal to 2.5 and that of oxygen is 3.5. Therefore, CO2 is a covalent molecule, non-polar specifically, however, it has a polar covalent bond (intramolecular force).Įrror message | View complete answer on What is CO2 compound name?Ĭarbon dioxide (chemical formula CO2) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms.Įrror message | View complete answer on Is so2 a covalent compound? Since there are four shared pair of electrons in the molecule of carbon dioxide, the number of covalent bonds formed will be four.Įrror message | View complete answer on Is carbon dioxide CO2 a covalent compound? Please comment below or contact us.In a carbon dioxide molecule, one carbon atom is joined by four covalent bonds to two oxygen atoms, each with two covalent bonds. The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged).Ī covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. What is chemical bond, ionic bond, covalent bond?Ī chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Answer: Al2O3 ( Aluminium oxide ) is ionic


 0 kommentar(er)
0 kommentar(er)
